Universal Biosensors
Xprecia Prime™
2023
Concept + Development
Xprecia Prime™
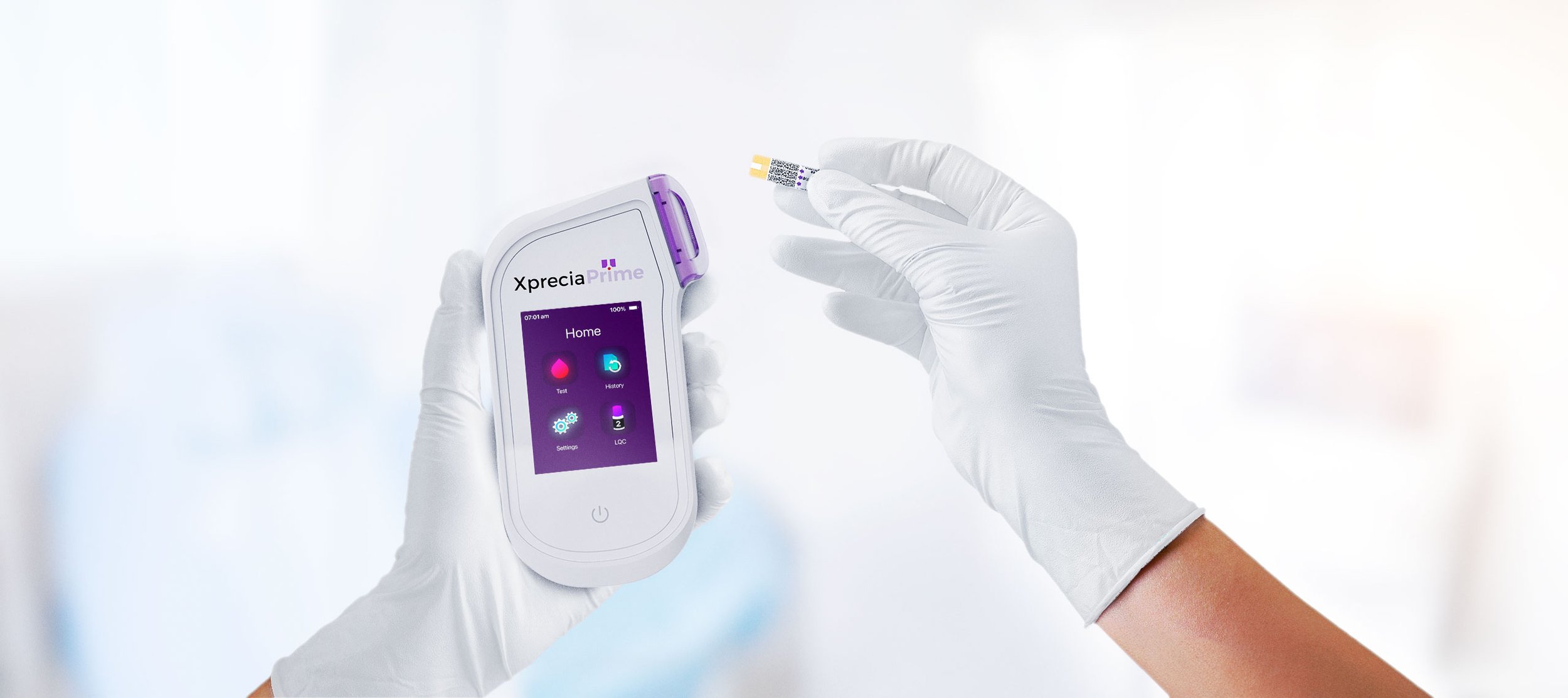
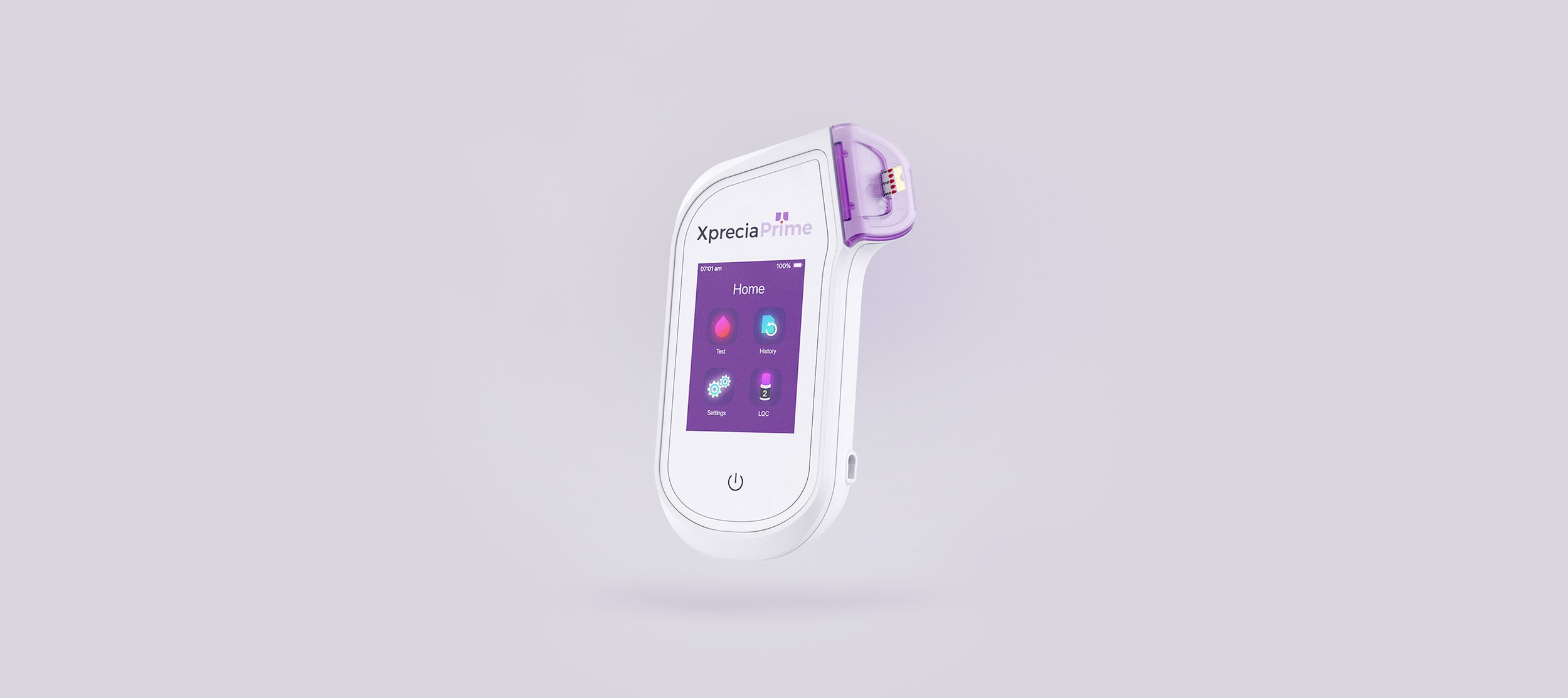
Portable Coagulation Analyser: The power of a lab in the palm of your hand.
The Universal Biosensors Xprecia Prime™ is an innovative portable coagulation monitoring device designed for fast and reliable prothrombin time (PT) and International Normalised Ratio (INR) testing, particularly for patients receiving vitamin K antagonist oral anticoagulation therapy, including widely used medications such as Warfarin and Coumadin. Building on the successful development of the Xprecia Stride® Coagulation Analyser device, our team focused on the mechanical design and integration of electronics architectures of the next-generation device, working alongside Universal Biosensors' internal software and electronics engineering teams. The analyser boasts a small hand-held form factor, and user-friendly interface, capable of providing PT/INR results in under a minute, closely resembling laboratory analysis. Xprecia Prime™ is a second-generation product that replaces Xprecia Stride® (First generation) improving on performance, connectivity and usability features.
Result in 5 simple steps, in under 1 minute
Approved for Self-testing in Europe: Empowering patients to conduct PT/INR testing at home.
Globally, approximately 10 million patients are taking warfarin, and over 200 million PT/INR tests are conducted annually to monitor the safe and effective dosage of anticoagulants. Providing instant results in various healthcare settings, including point of care (POC), primary care, urgent care, and hospitals, can assist in preventing adverse treatment outcomes. The PT/INR test allows physicians to appropriately adjust the patient’s dose of the drug to compensate for any diet and lifestyle changes. Xprecia Prime™ has received approval inder IVDR for Health Care Professional as well as Patient Self-Testing/ Near Patient testing (PST marketed separately as Xprecial Prime 4U). This approval has been issued by TÜV SÜD Product Service GmbH. This approval empowers patients to perform PT/INR testing at home, aligning with the growing European market for Patient Self-Testing, where the preference for approved self-testing solutions is on the rise.
For the US territory: Xprecia Prime is FDA 510k cleared and CLIA waived for Health Care Professional testing. Xprecia Prime 4U is not for sale in the USA.
To learn more, visit www.universalbiosensors.com