Alcolizer
Virulizer™ Diagnostic Platform
2022
Research, Concept, Development, Electronics + GUI
Alcolizer Virulizer™
A highly-sensitive rapid antigen COVID-19 mass-testing device — Discovered, Developed + Manufactured in Australia.
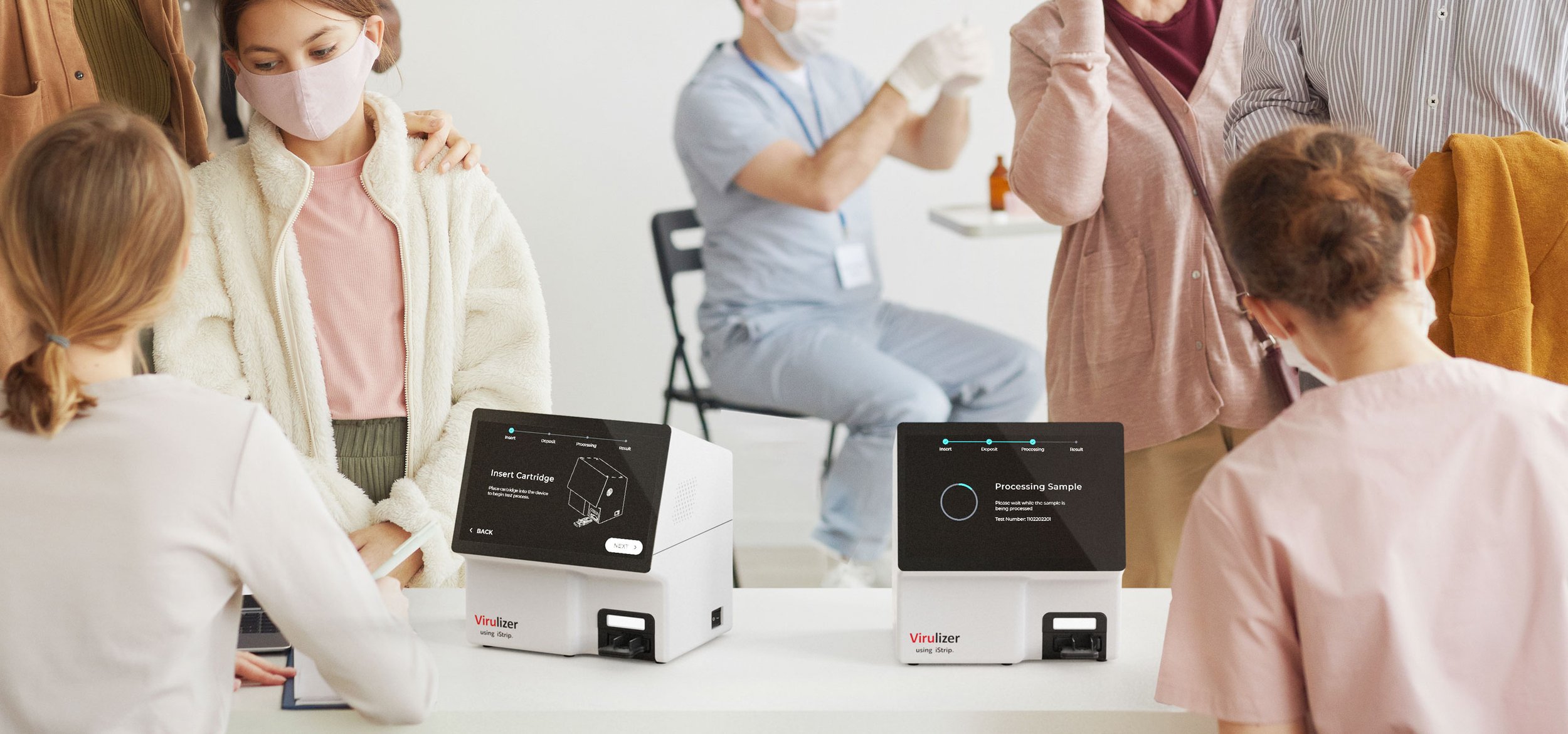
Alcolizer’s Virulizer™ — A highly-sensitive rapid antigen COVID-19 mass-testing device. Unlike other existing antigen and PCR tests, Virulizer™ is non-invasive, faster, cheaper and able to detect COVID-19 in under 10-minutes. Clinical study findings have confirmed that the Virulizer™ platform is at least 1000x more sensitive than commercially available antigen test kits currently on the market. The development is the result of collaboration between UTS, Alcolizer and D+I, with development funding provided by the Federal Government to support the technologies fast-tracking to market.
Highly Sensitive + Adaptable Platform
The sensitivity of the technology will be transformational to environments that require accurate and cost-effective rapid results.
Virulizer™ has been designed as an adaptable technology, with the intention to expand the platform to detect other infectious agents, proteins and metabolites at concentrations previously only possible using expensive clinical instruments. The sensitivity of the technology will be transformational to environments that require accurate and cost-effective rapid results. Additionally, the advanced technological Virulizer™ platform is an ideal solution for contact-tracing, employing cloud-based integration of results and location tracking — delivering real-time data analytics to target suburbs, pinpoint single results and monitor compliance in daily testing programs.
“Live trials are a crucial component of our commercialisation strategy, and Alcolizer with support from the Advanced Manufacturing Growth Centre and the Malaysian Investment Development Authority saw the potential to conduct clinical trials in Malaysia where an Emergency Use Pathway for medical devices is in place… Imagine being able to identify an asymptomatic carrier 2-3 days before they show any sign of illness in just ten minutes. Its application to industry, health care and travel is endless.” commented Roger Hunt, General Manager of Alcolizer.
Fast + Non-invasive Point-of-care Testing
How it works: A fast, non-invasive and easy to administer antigen test procedure.
Patient collections own saliva sample.
Patient deposits collector into lysis buffer.
Technician secures cap and waits 5-minutes.
Cartridge inserted into the desktop Virulizer™ detection instrument.
Technician deposits drops from buffer onto cartridge.
Test result displayed on detection device screen in under 10-minutes.
From Concept into Production
Research + Industry: Generating successful commercial and economic outcomes from R&D in Australia
Alcolizer, D+I and Lastek undertook the development from concept to delivery of functional devices for initial clinical trials in just 100 days. The rapid development consisted of the complete conceptual design + engineering development + delivery of an all-in-one diagnostic system. Areas of involvement included; product styling and design, tactile device + digital user interface development, improvements to laser + optical systems, improved camera selection, thermal management + integration of cooling systems, implementation of remote server access + enabling over-the-air updates.
Virulizer™ has the potential to save lives, as well as bring about real economic and job benefits for Australians — Alcolizer iStrips and testing instruments are to be manufactured at Alolizer’s fully automated robotic manufacturing facility in Balcatta, Western Australia. The development showcases the power of what can happen when you combine research and industry specialists to commercialise great ideas, seize new opportunities and solve key challenges.