Siemens
Xprecia Stride
2016
ISO 13485:2016 — Research, Concept, Development, Production, GUI + Packaging
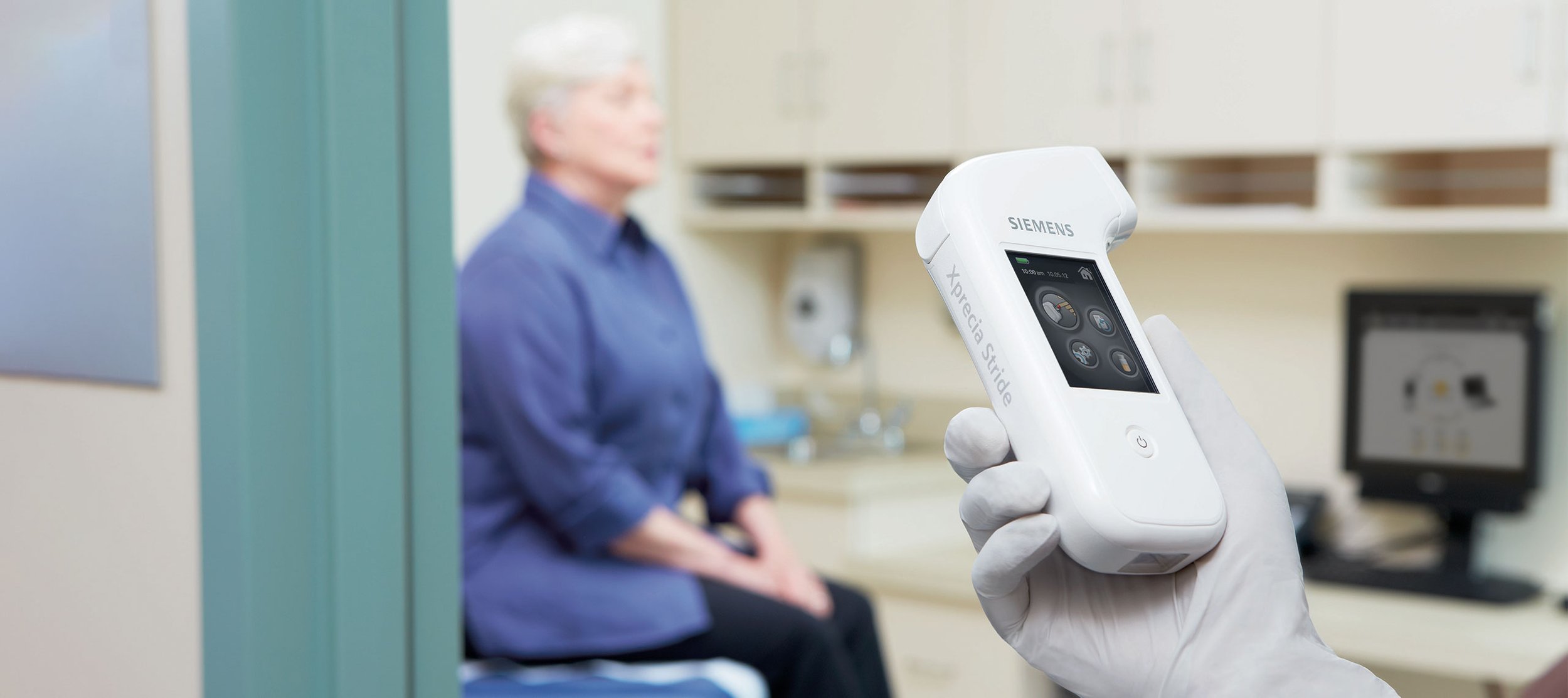
Siemens Xprecia Stride™
D+I worked with Siemens Healthineers and Universal Biosensors to develop the award-winning point-of-care (POC) blood coagulation analyser, the Xprecia Stride™. The Xprecia Stride™ Coagulation system offers significant advantages in patient care, with a modern ergonomic design and a superior user experience designed to improve point-of-care blood coagulation testing. All system elements integrate good design principles, usability, and state-of-the-art enhancements to improve the testing experience for both patients and professionals.
Cutting edge diagnostics made personal.
Xprecia Stride™ incorporates cutting edge Siemens’ hemostasis technology and Universal Biosensors’ electrochemical biosensors strip technology in a ground-breaking point-of-care device that delivers lab-quality results. Its compact form factor, ease-of-use and quality of results enables more frequent monitoring as well as urgent emergency-care monitoring of blood clotting tendencies in patients. By measuring the ratio of prothrombin time over international normalised ratio (PT/INR), oral anticoagulation therapy (OAT) can be managed by the patient-care team. The combination of strip design, sensors, hardware and software deliver a suite of technologies that create the unique value proposition of this system.
Optimised Usability + Operability
The analyzer's design incorporates a comprehensive graphical user interface to guide the user during the testing process. Its simplicity in physical design and the incorporation of a strip ejection mechanism eliminates the need for the user to touch bloody used test strips. Furthermore, the device has a unique feature in its test strip pop-off cap. The test strip protective cap includes a series of carefully designed and engineered capillary channels. In a low probability “flooding” event of a larger than usual blood sample, these capillaries immediately draw the blood away from the electronics on the test strip port. The protective cap can be easily removed for cleaning.
Stringent medical safety and performance standards
The product uses best-in-class materials to meet its stringent medical safety and performance standards. The electronics are all lead-free and ROHS compliant. All plastic resins and metal parts and their associated manufacturing processes all include optimized solutions. Significant investment and R&D resources have gone into the engineering of the device's plastic enclosure. It is one of the first blood-handling point-of-care medical devices to meet the latest stringent FDA regulation in disinfection protocol for devices that handles contaminated blood (including HIV-infected blood). Through a combination of materials and product engineering, the enclosure has very high performance in sustained frequent chemical wipe down. It is designed to last many years in professional and hospital conditions without environmental stress cracking or material failure. The achieved validated performance outcome has come from extensive internal product reliability testing.